Comentaries on Prion Diseases and Ozoneby Gérard V. Sunnen, M.D.A number of diseases afflicting humans, animals and plants are, in contemporary times, stimulating great interest. This is due to the fact that these conditions do not follow traditional trajectories of infectivity, and that the responsible infective agents have poorly understood structural configurations and/or mechanisms of action. Transmissible Spongiform Encephalopathies (TSEs) occur in humans and animals, and are characterized by progressive pathological effects upon the nervous system, invariably resulting in death. As their names suggest, they produce, usually over long periods of time, neuronal loss and gliosis, leading to microlacunae in brain tissue, which are apt to fill, by unknown mechanisms, with amyloid-like deposits. Human TSEs include Creuzfeldt-Jacob disease (CJD), Familial fatal insomnia (FFI), Kuru, and Gerstmann-Straussler-Scheinker disease (GSS). Animal TSEs include Scrapie in sheep, Feline spongiform encephalopathy (FSE), Chronic wasting disease in deer and ungulates, and of most concern in current times, Bovine spongiform encephalopathy (BSE). This concern is derived from evidence of transmission of TSEs from animals to humans. These diseases were suspected of having viral origins, until the 1960's when T Alper suggested that the scrapie agent might have the capacity of replicating without the presence of nucleic acid. In 1982, S Prusiner coined the word prion, to stand for a new class of infective agents with highly novel characteristics. Prions are not conventional viruses. Nucleic acid is apparently not necessary for their infectivity. In addition, they appear to interact with host genes, cell proteins, and cellular strucures in very complex and yet unchartered fashion. Properties which further differentiate prions from viruses include their resistance to radiation, to heat inactivation, to DNAse and RNAse treatment, and to such protein-denaturing chemicals as phenols. These characteristics point to a protein structure in prions. Some prions have been shown to consist of glycoproteins with amino acid sequences approximating 250 units. There is no evidence at this time that prions contain lipid components. It appears, interestingly, that, aside from the precise sequencing of amino acids within their structure, the spatial configuration of prions may play an important role in their pathogenecity, especially as it relates to the disruption of neuronal membranes. There is evidence that this is true in the case of scrapie prions which contain two highly hydrophobic regions capable of spanning such membranes. Presumably, an alteration in the molecular architecture of the prion could impair this capacity for membrane attachement, and thus compromise its destructive potential. Prions, by all current evidence, are very precisely structured molecules, whose specific stereotaxis needs to be quintessentially intact for the expression of their infectivity. Ozone has not yet been studied for the inactivation of prions or viroids (such as HDV). While the agents cited above such as heat, radiation, enzymes, and cleaving chemicals, have proven to be unsuccessful in inactivating prions, ozone has properties which are sufficiently distinct from these agents to embody a unique potential in offering novel mechanisms of prion destruction. Specifically, ozone has the proven ability to offer intense oxidizing action, which may provide a means of altering the prion's precisely tuned chemical and spatial design through:
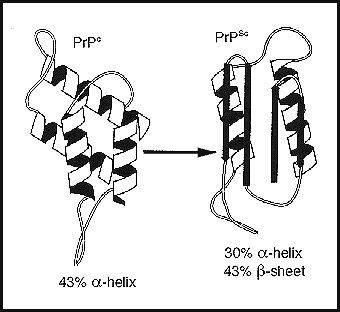
From: Cann A J 1997 Principles of Molecular Virology. Academic Press, San DiegoThe illustration shows two different spatial configurations of the same prion structure. The one on the right is infectious and pathogenic, while the other is not. The factors which promote prions to metamorphose their spatial architecture are unknown. In conclusion, ozone offers unique capabilities to potentially destroy prions. Ozone, while altering these small infective protein molecules, is theoretically capable of exerting its contrapathogenic role, while leaving much larger and versatile protein and lipid molecules found in mammalian serum functionally intact. BIBLIOGRAPHY
|